We invite you to an exclusive free trial of our all-new global-standard
self-study course in clinical research methods, including:
5 engaging slideshow lessons
with narrations and animated video
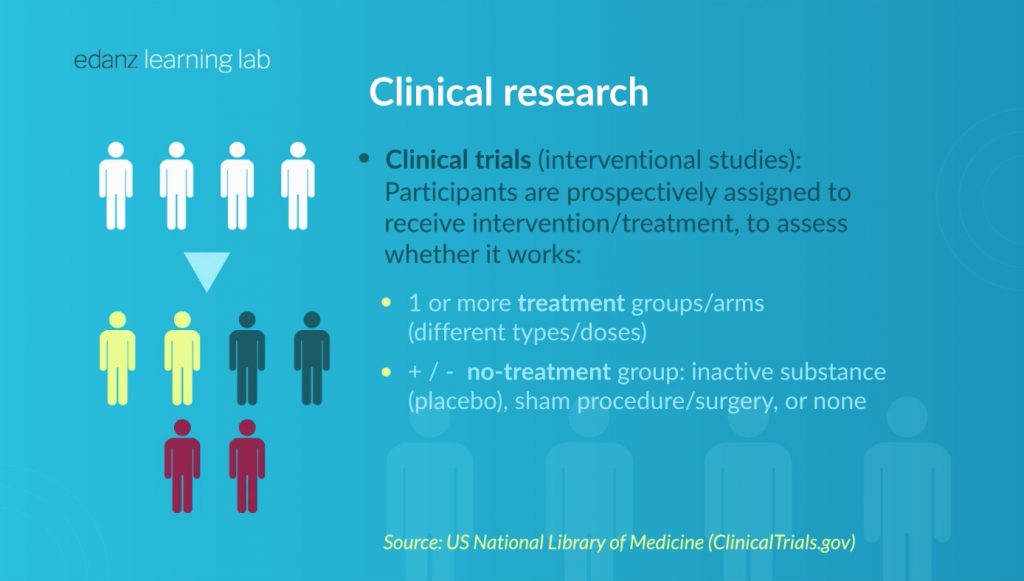
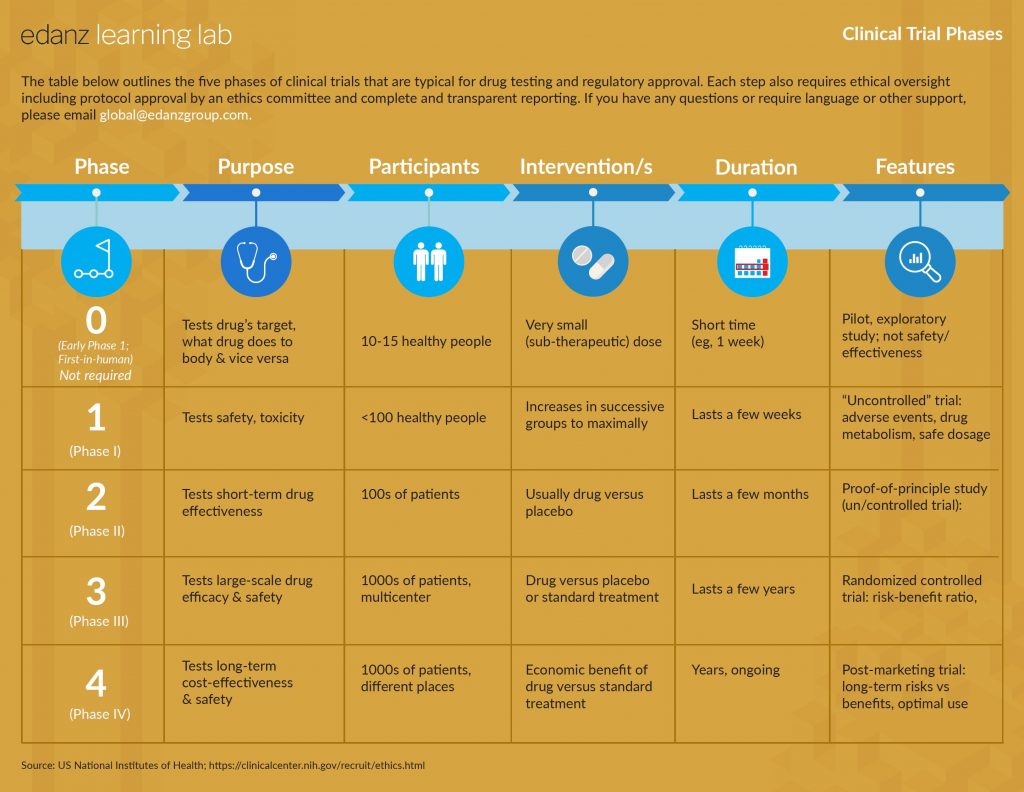
1 • Clinical trials and guidelines
2 • Good clinical practices
3 • Trial protocol records and reporting
4 • Research questions and objectives
5 • Study types, methods, and data analysis
15 colorful PDF mini-guides
packed with useful tips, tricks,
templates, and checklists
5 assessment quizzes
• certification of completion
• group progress dashboard for institutional administrators
Try the full course for free:
If you already have an Edanz account, click “Log In” now, then go to Step 2 below.
If you don’t yet have a MY edanz account, click “CREATE ACCOUNT” at the top of this page. After signing up, check your email inbox for the message, “Please Verify Your Account”. Click on the link to verify. You will be taken to your new MY edanz account. Welcome!
Come back here to Learning Lab and click “Sign In“, then go on to Step 2.
Please sign in / sign up first (Step 1)
In the top menu, go to “Courses” and click “MY Learning Lab” to see your courses and track your progress. You can also find more free courses on the ‘All Courses’ page. Enjoy learning to maximize your clinical research impact!
Developed and presented by top research professionals
with a deep understanding of and commitment to
ethical clinical research principles and practices

Dr. Trevor Lane
• Postdoctoral research positions in clinical oncology and genetics
• a variety of senior editorial roles in academic, research, and publishing institutions
• managing editor of several medical journals in Asia and senior editor of two journals in the USA
• helping researchers publish their research results internationall for over 20 years

Dr. Dean Meyer
• Dr Meyer has a background in environmental science with a specialist interest in toxicology and public health.
• She spent eight years working at the Centers for Disease Control and Prevention in Atlanta, and has an extensive background in the areas of laboratory safety and environmental health.

Dr. Matt Glasgow
• Dr Matt Glasgow (MBChB, PhD) specializes in health economics, including cost-utility/cost-benefit analysis, and decision analytic modelling.
•His background includes clinical practice in a variety of medical fields, particularly sports medicine, and postgraduate health informatics qualifications and experience including the development and maintenance of assorted clinical decision support technologies.

Dr. Daniel McGowan
• PhD Molecular Neuroscience, University of Auckland
• Certified Medical Publications Professional (CMPP) and ISMPP member
• Served as Senior Scientist at the University of Cambridge, UK and as a Marie Curie Research Fellow at the Babraham Institute in Cambridge
Who are Edanz?
Edanz has helped tens of thousands of researchers around the world
to successfully publish in leading global journals for more than 25 years

Questions? We love questions! Contact us